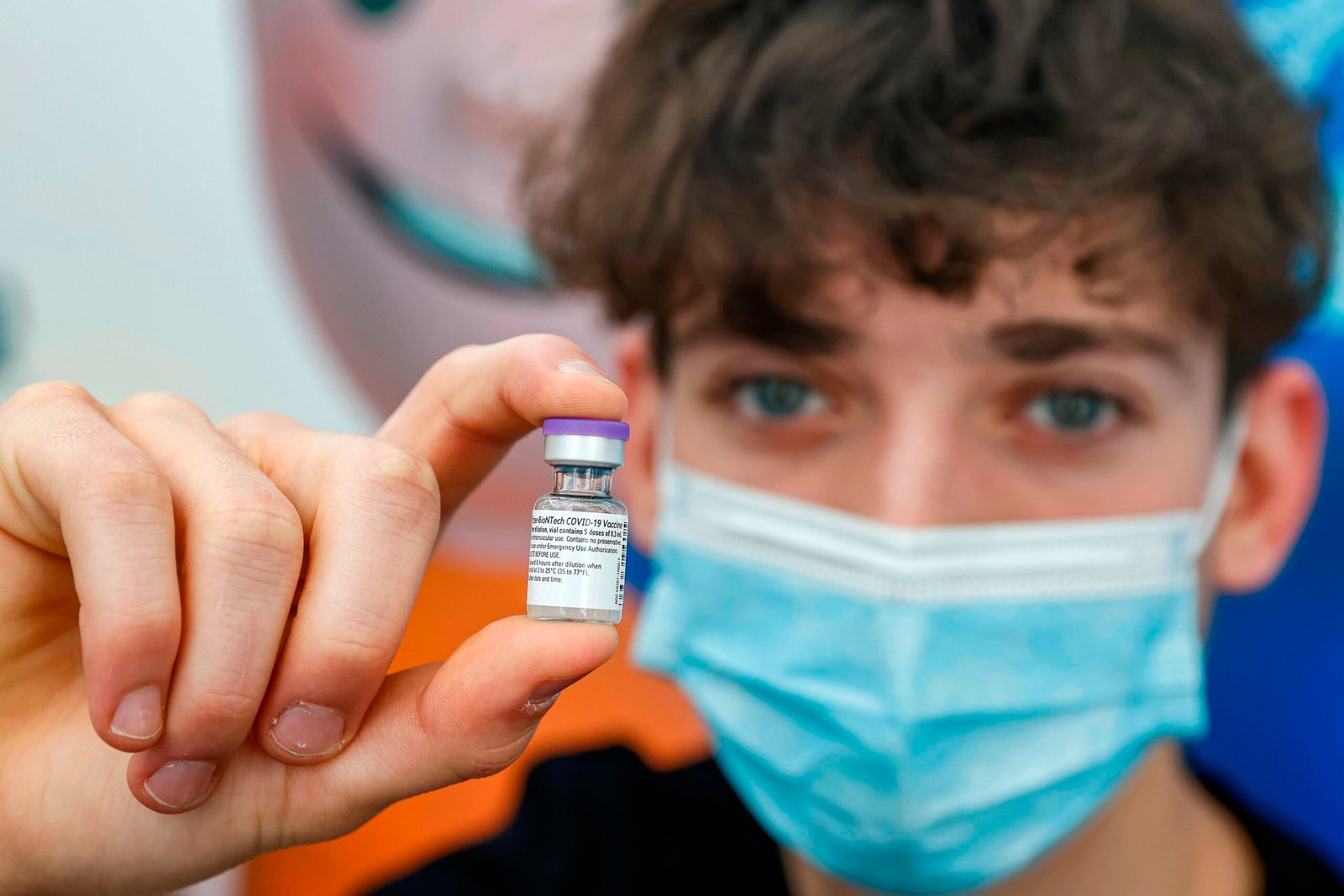
A 16-year-old teenager, receives a dose of the Pfizer-BioNtech COVID-19 coronavirus vaccine.
Jack Guez | AFP | Getty Photographs
The Meals and Drug Administration on Thursday expanded eligibility for Pfizer and BioNTech’s booster shot to people who find themselves 16 and 17 years previous at the very least six months after their main vaccination.
The FDA’s emergency authorization comes a day after Pfizer and BioNTech launched preliminary lab information indicating that booster photographs present excessive ranges of safety towards the extremely mutated omicron variant of the virus that causes Covid-19.
The preliminary information discovered that omicron considerably reduces the safety supplied by the preliminary two-dose collection. Boosters, however, struggle the variant at ranges corresponding to the 95% safety supplied by the two-dose collection towards the unique pressure of the virus, the lab outcomes confirmed.
“As folks collect indoors with household and pals for the vacations, we will not let up on all of the preventive public well being measures that we’ve been taking throughout the pandemic,” appearing FDA Commissioner Janet Woodcock stated in a press launch. “With each the delta and omicron variants persevering with to unfold, vaccination stays one of the best safety towards COVID-19.”
Regulators within the U.S. have been extra hesitant approve vaccines for youthful folks attributable to considerations about myocarditis, a uncommon inflammatory coronary heart situation that is been discovered most prevalent in younger males and regarded as associated to testosterone ranges.
The FDA on Thursday stated it decided the advantages of the photographs outweighed the dangers of myocarditis after evaluating extra real-world information.
The most typical signs reported by individuals who have obtained boosters are ache, redness and swelling on the injection web site in addition to fatigue, muscle and joint level and chills, in line with the FDA.
The Facilities for Illness Management and Prevention nonetheless must authorize boosters for 16- and 17-year-olds earlier than the photographs are administered. The CDC cleared booster photographs for all adults 18 and over final month.
Public well being authorities within the U.S. are calling for everybody who’s eligible to get a booster shot, amid fears of a winter Covid surge pushed by the delta variant and uncertainty concerning the future course of the pandemic attributable to omicron.
The efficacy of the preliminary two-dose vaccination collection was declining earlier than the arrival of omicron. The journal Science printed a research final month that discovered the effectiveness of Pfizer’s two-dose vaccine declined to 43% from 86% from February to October of this 12 months.
The vice chairman of Pfizer’s vaccine scientific analysis program, Dr. John Perez, informed a CDC advisory committee final month that the booster was 95% efficient at stopping symptomatic an infection in a scientific trial of 10,000 contributors 16 years and older.
















 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  XRP
XRP  USDC
USDC  Solana
Solana  TRON
TRON  Lido Staked Ether
Lido Staked Ether  Dogecoin
Dogecoin  Figure Heloc
Figure Heloc  Bitcoin Cash
Bitcoin Cash  WhiteBIT Coin
WhiteBIT Coin  Cardano
Cardano  USDS
USDS  Wrapped stETH
Wrapped stETH  LEO Token
LEO Token  Hyperliquid
Hyperliquid  Wrapped Bitcoin
Wrapped Bitcoin  Chainlink
Chainlink  Binance Bridged USDT (BNB Smart Chain)
Binance Bridged USDT (BNB Smart Chain)  Ethena USDe
Ethena USDe  Canton
Canton  Monero
Monero  Stellar
Stellar  Wrapped eETH
Wrapped eETH  USD1
USD1  Rain
Rain  sUSDS
sUSDS  Zcash
Zcash  Hedera
Hedera  Litecoin
Litecoin  Coinbase Wrapped BTC
Coinbase Wrapped BTC  Dai
Dai  PayPal USD
PayPal USD  Avalanche
Avalanche  WETH
WETH  Shiba Inu
Shiba Inu  Sui
Sui  World Liberty Financial
World Liberty Financial  USDT0
USDT0  Toncoin
Toncoin  Cronos
Cronos  Tether Gold
Tether Gold  MemeCore
MemeCore  PAX Gold
PAX Gold  Uniswap
Uniswap  Polkadot
Polkadot  Ethena Staked USDe
Ethena Staked USDe  Mantle
Mantle  BlackRock USD Institutional Digital Liquidity Fund
BlackRock USD Institutional Digital Liquidity Fund  Aave
Aave  Aster
Aster  Falcon USD
Falcon USD  Pepe
Pepe  Bittensor
Bittensor  OKB
OKB  Bitget Token
Bitget Token  Global Dollar
Global Dollar  Circle USYC
Circle USYC  syrupUSDC
syrupUSDC  HTX DAO
HTX DAO  Ripple USD
Ripple USD  Pi Network
Pi Network  Sky
Sky  Ethereum Classic
Ethereum Classic  NEAR Protocol
NEAR Protocol  BFUSD
BFUSD  Ondo
Ondo  Pump.fun
Pump.fun  Superstate Short Duration U.S. Government Securities Fund (USTB)
Superstate Short Duration U.S. Government Securities Fund (USTB)  Internet Computer
Internet Computer  POL (ex-MATIC)
POL (ex-MATIC)  Cosmos Hub
Cosmos Hub  Gate
Gate  KuCoin
KuCoin  Jupiter Perpetuals Liquidity Provider Token
Jupiter Perpetuals Liquidity Provider Token  Worldcoin
Worldcoin  Midnight
Midnight  Quant
Quant  NEXO
NEXO  Jito Staked SOL
Jito Staked SOL  Ethena
Ethena  USDtb
USDtb  Binance-Peg WETH
Binance-Peg WETH  Rocket Pool ETH
Rocket Pool ETH  Official Trump
Official Trump  Algorand
Algorand  Binance Bridged USDC (BNB Smart Chain)
Binance Bridged USDC (BNB Smart Chain)  Spiko EU T-Bills Money Market Fund
Spiko EU T-Bills Money Market Fund  USDD
USDD  Wrapped BNB
Wrapped BNB  Render
Render  Function FBTC
Function FBTC
GIPHY App Key not set. Please check settings