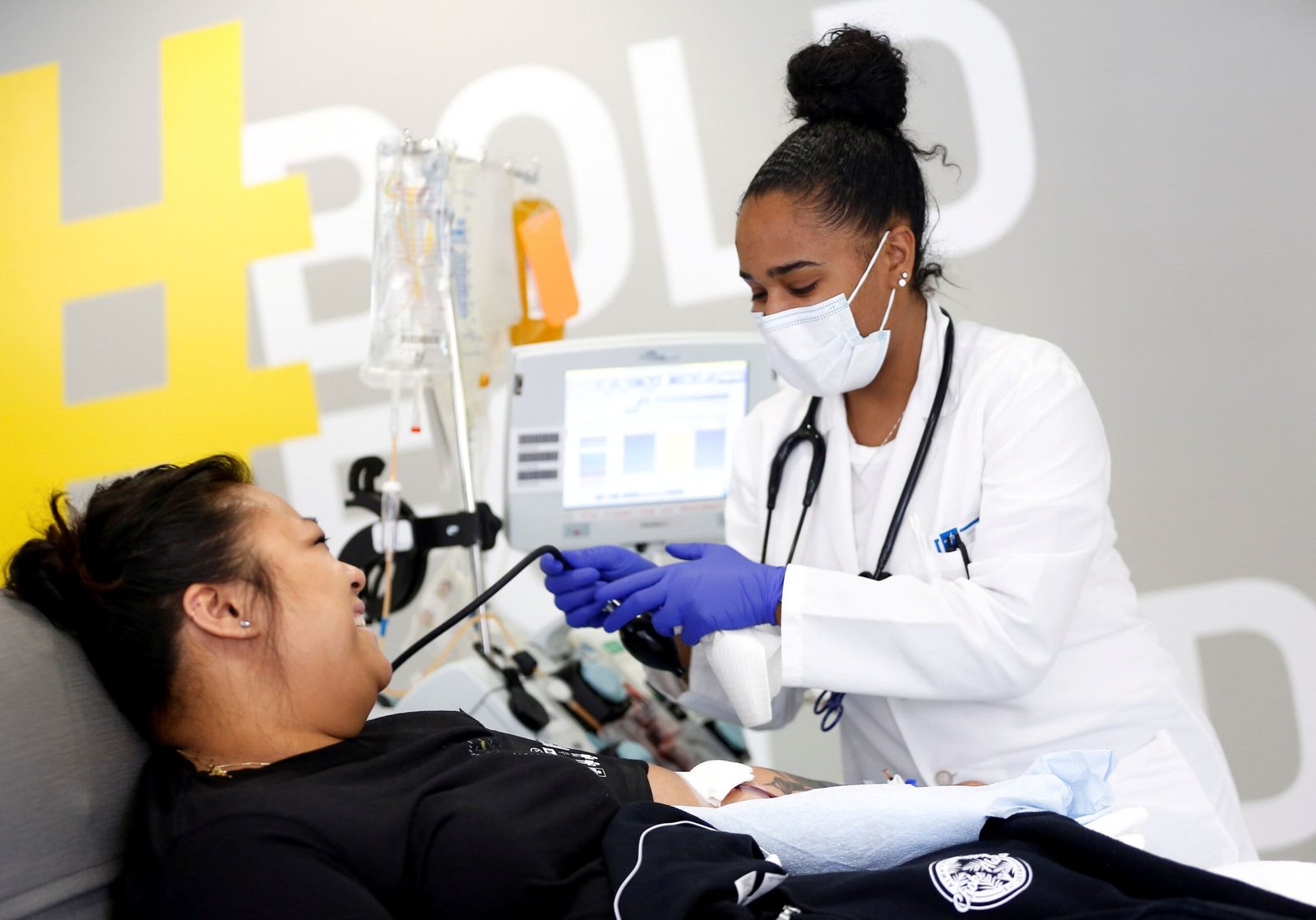
Blood assortment specialist Kathryn Severson holds a bag of convalescent plasma from a recovered coronavirus affected person on the Central Seattle Donor Middle of Bloodworks Northwest throughout the coronavirus illness (COVID-19) international outbreak, in Seattle, Washington, September 2, 2020.
Lindsey Wasson | Reuters
The World Well being Group on Monday issued a robust advice towards administering convalescent plasma to deal with Covid-19 sufferers, citing analysis that reveals no enchancment in sufferers who acquired the remedy.
In convalescent plasma remedy, blood plasma is donated by somebody who has recovered from the virus and transferred right into a affected person battling the virus with the hope the donor’s antibodies assist struggle the an infection.
Nonetheless, the WHO’s guideline improvement group discovered that “there was no clear profit for important outcomes corresponding to mortality and mechanical air flow for sufferers with non-severe, extreme or important sickness, and important useful resource necessities when it comes to price and time for administration.”
The group stated the remedy additionally faces sensible challenges, corresponding to discovering and testing donors in addition to gathering, storing and transporting the plasma.
The advice relies on 16 trials with greater than 16,000 sufferers with non-severe, extreme and demanding Covid infections. The group stated analysis on the remedy ought to proceed in randomized management trials. The brand new advice is printed within the British Medical Journal.
The U.S. Meals and Drug Administration in February scaled again its convalescent plasma emergency use authorization to cowl solely hospitalized sufferers early in illness development and people hospitalized who’ve immune system problems wherein they can not produce a robust antibody response.
“Plasma with low ranges of antibodies has not been proven to be useful in COVID-19,” the FDA stated in its revised emergency authorization in February.
The company issued its unique, broader authorization on an emergency foundation within the U.S. for all hospitalized sufferers in August 2020 when there have been no different permitted remedies for the virus. Throughout the Trump administration, Well being and Human Companies Secretary Alex Azar had celebrated the usage of convalescent plasma on the time as a “milestone achievement” within the efforts to struggle Covid.
Since then, the FDA has licensed two Covid remedies: Gilead’s antiviral drug remdesivir in October 2020 and Regeneron’s antibody cocktail the next month. Pfizer’s two-dose vaccine was licensed a couple of yr in the past.
The Nationwide Institutes of Well being in August additionally stated convalescent plasma did not assist sufferers in an NIH-backed examine of greater than 500 grownup Covid sufferers on the College of Pittsburgh. The trial was stopped in February as a result of its lack of effectiveness, the NIH stated.
The New England Journal of Medication, in a examine printed final month, discovered that convalescent plasma didn’t stop illness development in high-risk outpatients when administered one week after symptom onset. It additionally didn’t enhance scientific outcomes in hospitalized sufferers late in the midst of their sickness, in line with the examine.
Nonetheless, the examine discovered that convalescent plasma did scale back illness development in older, outpatient adults if administered inside 72 hours of symptom onset.
Pfizer and Merck are actually searching for emergency use authorization for oral antiviral medication designed to scale back the chance of hospitalization from Covid.
















 Bitcoin
Bitcoin  Ethereum
Ethereum  Tether
Tether  Solana
Solana  USDC
USDC  XRP
XRP  Lido Staked Ether
Lido Staked Ether  Dogecoin
Dogecoin  Toncoin
Toncoin  Cardano
Cardano  TRON
TRON  Avalanche
Avalanche  Wrapped Bitcoin
Wrapped Bitcoin  Shiba Inu
Shiba Inu  Chainlink
Chainlink  Polkadot
Polkadot  Bitcoin Cash
Bitcoin Cash  NEAR Protocol
NEAR Protocol  Uniswap
Uniswap  LEO Token
LEO Token  Litecoin
Litecoin  Dai
Dai  Pepe
Pepe  Wrapped eETH
Wrapped eETH  Polygon
Polygon  Internet Computer
Internet Computer  Aptos
Aptos  Ethereum Classic
Ethereum Classic  Ethena USDe
Ethena USDe  Artificial Superintelligence Alliance
Artificial Superintelligence Alliance  Stellar
Stellar  Monero
Monero  Stacks
Stacks  Mantle
Mantle  Render
Render  Filecoin
Filecoin  dogwifhat
dogwifhat  OKB
OKB  Bittensor
Bittensor  Injective
Injective  Hedera
Hedera  Maker
Maker  Cronos
Cronos  Cosmos Hub
Cosmos Hub  Arbitrum
Arbitrum  Immutable
Immutable  Arweave
Arweave  Bonk
Bonk  First Digital USD
First Digital USD  Sui
Sui  Optimism
Optimism  The Graph
The Graph  Rocket Pool ETH
Rocket Pool ETH  FLOKI
FLOKI  Renzo Restaked ETH
Renzo Restaked ETH  Mantle Staked Ether
Mantle Staked Ether  THORChain
THORChain  Jupiter
Jupiter  Theta Network
Theta Network  Aave
Aave  Notcoin
Notcoin  JasmyCoin
JasmyCoin  WhiteBIT Coin
WhiteBIT Coin  Ondo
Ondo  Pyth Network
Pyth Network  Lido DAO
Lido DAO  Brett
Brett  Fantom
Fantom  Core
Core  Celestia
Celestia  Sei
Sei  Algorand
Algorand  ether.fi Staked ETH
ether.fi Staked ETH  Quant
Quant  Flow
Flow  Gate
Gate  Marinade Staked SOL
Marinade Staked SOL  MANTRA
MANTRA  Beam
Beam  KuCoin
KuCoin  MultiversX
MultiversX  Bitcoin SV
Bitcoin SV  Axie Infinity
Axie Infinity  Helium
Helium  Popcat
Popcat  Ethereum Name Service
Ethereum Name Service  GALA
GALA  EOS
EOS  BitTorrent
BitTorrent  Tokenize Xchange
Tokenize Xchange  ORDI
ORDI  NEO
NEO  Akash Network
Akash Network  dYdX
dYdX
GIPHY App Key not set. Please check settings